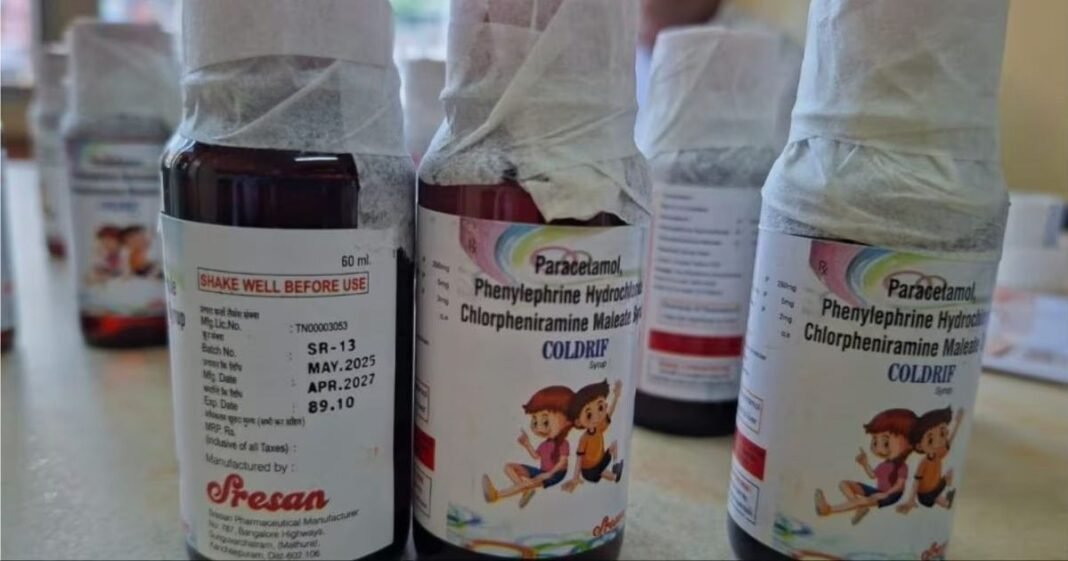
The World Health Organization has expressed serious concerns regarding India’s drug safety regulations following reports of at least 20 children succumbing to tainted cough syrups. Tragic deaths in Madhya Pradesh and Rajasthan have been linked to three specific cough syrups containing the toxic substance diethylene glycol (DEG), commonly found in industrial solvents.
Identified as Coldrif (Sresan Pharmaceuticals), Respifresh (Rednex Pharmaceuticals), and ReLife (Shape Pharma), the contaminated syrups have had their production halted, with an official investigation initiated by Indian drug authorities. The WHO has cautioned that these dangerous cough syrups could potentially infiltrate other countries through unregulated distribution channels.
The WHO emphasized the lethal nature of DEG, underscoring that even small amounts of the substance can be fatal, particularly for children. The organization stressed that diethylene glycol (DEG) and ethylene glycol (EG) are not meant for human consumption and are typically utilized in industrial products like antifreeze and adhesives.
In a recent development, G Ranganathan, the 73-year-old owner of Sresan Pharmaceuticals, was arrested, and the company’s manufacturing license is set to be permanently revoked according to Tamil Nadu Health Minister Ma Subramaniam. An inspection by the drug department revealed that Sresan Pharmaceuticals had breached 364 manufacturing regulations, citing issues such as unqualified staff, poor pest control, and unsanitary storage of manufactured products.
This unfortunate incident is not the first instance of concerns surrounding cough syrups manufactured in India. In previous cases, a significant number of children in Gambia and Uzbekistan lost their lives due to tainted cough syrups sourced from India. India, ranked as the third-largest drug producer globally after the United States and China, typically screens pharmaceutical products for DEG using gas chromatography. However, many laboratories in less-resourced countries lack access to this technology, as highlighted by the WHO.
Earlier this year, the WHO initiated a public consultation on screening devices to detect DEG-contaminated medications. The organization stressed the urgency of addressing the presence of this harmful substance in pediatric syrups, which has led to fatal outcomes in various countries, especially among children. DEG and EG have been linked to severe health complications, including multiorgan failure, acute renal failure, and neurological dysfunction, resulting in numerous deaths, especially among children.